Metal complexes and metalloproteases: targeting conformational diseases
Abstract
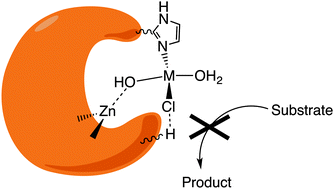
In recent years many metalloproteases (MPs) have been shown to play important roles in the development of various pathological conditions. Although most of the literature is focused on matrix MPs (MMPs), many other MPs have been demonstrated to be involved in the degradation of peptides or proteins whose accumulation and dyshomeostasis are considered as being responsible for the development of conformational diseases, i.e., diseases where non-native protein conformations lead to protein aggregation. It seems clear that, at least in principle, it must be possible to control the levels of many aggregation-prone proteins not only by reducing their production, but also by enhancing their catabolism. Metal complexes that can perform this function were designed and tested according to at least two different strategies: (i) intervening on the endogenous MPs by directly or indirectly modulating their activity; (ii) acting as artificial MPs, replacing or synergistically functioning with endogenous MPs. These two different bioinorganic approaches are widely represented in the current literature and the aim of this review is to rationally organize and discuss both of them so as to give a critical insight into these approaches and highlighting their limitations and future perspectives.

 Please wait while we load your content...
Please wait while we load your content...