Biological evaluation and molecular modelling of didanosine derivatives†
Abstract
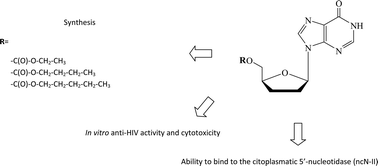
Five carbonate derivatives of 5′-O-2′,3′-dideoxyinosine (DDI, 1) have been synthesized by combination with aliphatic alcohols, with their in vitro anti-HIV activity and cytotoxicity being evaluated afterward in human peripheral blood mononuclear cells (PBMCs). One particular compound, namely DDI-Penta, exhibited an outstanding performance because it was found to have both a higher inhibitory potency and a lower cytotoxicity than the lead compound, resulting in a 100× enhancement in its selectivity index. In order to further study this phenomenon, the ability of these derivatives to bind to the cytoplasmatic 5′-nucleotidase (ncN-II) was studied by in silico methods. Also, the higher calculated lipophilicity of the synthesized compounds was proposed to improve their permeability through the cell membrane since said lipophilicity would allow a higher concentration of the corresponding prodrug inside the infected cell. Overall, a combination of an optimal lipophilicity and the ability of DDI-Penta to bind to ncN-II is suggested due to the higher potency and lower cytotoxicity observed for this compound. Based on the reported findings, we believe that the combination of certain aliphatic alcohols and DDI through a carbonate linkage could significantly increase the performance of this class of therapeutic compounds; therefore, it merits further evaluations.

 Please wait while we load your content...
Please wait while we load your content...