Design, synthesis and evaluation of N6-substituted 2-aminoadenosine-5′-N-methylcarboxamides as A3 adenosine receptor agonists†
Abstract
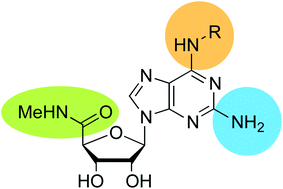
A series of N6-substituted 2-aminoadenosine-5′-N-methylcarboxamides were synthesized from the versatile intermediate, O6-(benzotriazol-1-yl)-2-amino-2′,3′-O-isopropylideneinosine-5′-N-methylcarboxamide (1). These compounds were evaluated as A3 adenosine receptor agonists in terms of their potency and receptor sub-type selectivity in a cAMP accumulation assay and a number of potent and selective compounds were identified. One such compound, 2-amino-N6-(3-chlorobenzyl)adenosine-5′-N-methylcarboxamide (3i), was over 500-fold selective for the A3AR over the other three adenosine receptor subtypes. This represents a significantly greater selectivity profile compared with the prototypical IB-MECA.

 Please wait while we load your content...
Please wait while we load your content...