Rapid in vitro protein synthesis pipeline: a promising tool for cost-effective protein array design
Abstract
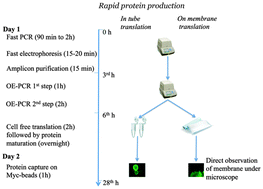
Several protein expression systems for construction of protein arrays have been established in recent years. However, current protocols for protein synthesis are still time consuming, laborious and expensive. This study has established an alternative workflow that covers rapid construction of expression cassettes, in-tube and on-membrane synthesis of recombinant proteins, and straightforward screening of synthesized proteins. Eighteen membrane associated eukaryotic proteins and two secretory complement regulators (C1 inhibitor and vitronectin) were included in the study. To generate hybrid genes, double-overlap extension PCR was employed to fuse the 5′ fragment (consisting of a T7 promoter and a species independent translation sequence), ORFs of the target proteins, and the 3′ fragment (encompassing GFP fusion, Myc-tag and stop codon). OE-PCR generated fragments were directly mixed with the Leishmania torentolae lysate (translation mix) for protein synthesis. In order to establish a cheap and user-friendly alternative to existing cell-free protein array techniques, PCR products were spotted on the hydrophobic substrate (PVDF membrane), air-dried and covered with only 2 μL of translation mix. All synthesized proteins were spontaneously immobilized on the membrane due to the hydrophobic interaction between C-terminally fused GFP and PVDF. Synthesis and immobilization of proteins were confirmed simply by assessing the GFP chromophore under a laser scanner or a fluorescent microscope.
- This article is part of the themed collection: Italian Proteomics Association, 2014

 Please wait while we load your content...
Please wait while we load your content...