Ammonia-free coprecipitation synthesis of a Ni–Co–Mn hydroxide precursor for high-performance battery cathode materials
Abstract
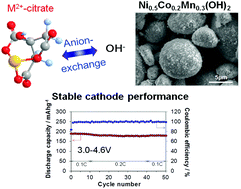
The ammonia-free green coprecipitation process has successfully produced a micro-sized spherical Ni0.5Co0.2Mn0.3(OH)2 precursor with a homogeneous elemental distribution. The replacement of ammonia with citric acid as a chelating agent was the key for this eco-friendly and cost-effective process. The pH of coprecipitation was determined from solubility and complex formation diagrams calculated using solubility products and formation constants. By varying the relative ratio of citric acid to metals, the optimized particle morphology, size and robustness of the precursor were obtained. The cathode material LiNi0.5Co0.2Mn0.3O2, prepared using the hydroxide coprecipitate precursor, showed a good charge–discharge cycling performance in lithium cells, delivering high capacity and cycling stability (≥94% retention) at 3.0–4.3 V as well as upon high-voltage operation at 4.6 V.

 Please wait while we load your content...
Please wait while we load your content...