Speciation and structure of tin(ii) in hyper-alkaline aqueous solution†
Abstract
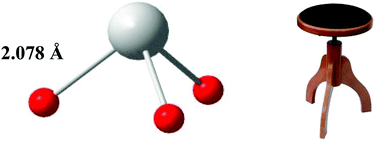
The identity of the predominating tin(II)–hydroxide complex formed in hyper-alkaline aqueous solutions (0.2 ≤ CNaOH ≤ 12 mol dm−3) is determined by potentiometric titrations, Raman, Mössbauer and XANES spectroscopy, supplemented by quantum chemical calculations. Thermodynamic studies using a H2/Pt electrode up to free hydroxide concentrations of 1 mol dm−3 showed the presence of a single monomeric complex with a tin(II) : hydroxide ratio of 1 : 3. This observation together with Raman and Mössbauer spectroscopic measurements supplemented by quantum mechanical calculations proved that the predominating complex is [Sn(OH)3]−, and that the presence of the other possible complex, [SnO(OH)]−, could not be proven with either experiments or simulations. The structure of the trihydroxidostannate(II) complex, [Sn(OH)3]−, was determined by EXAFS and was found to be independent of the applied hydroxide and tin(II) concentrations. The mean Sn–O bond distance is short, 2.078 Å, and in very good agreement with the only structure reported in the solid state. It is also shown that at pH values above 13 the speciation of the predominant trihydroxidostannate(II) complex is not affected by the presence of high concentrations of chloride ions.

 Please wait while we load your content...
Please wait while we load your content...