Half sandwich ruthenium(ii) hydrides: hydrogenation of terminal, internal, cyclic and functionalized olefins†
Abstract
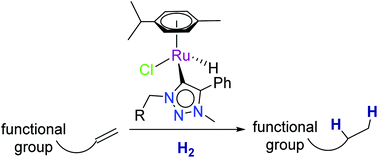
Bis(1,2,3-triazolylidene) silver(I) complex 1a was reacted with [RuCl2(p-cymene)]2 to give the ruthenium complex [PhCH2N2(NMe)C2(C6H4CF3)]RuCl2(p-cymene) (2a) as major product in addition to the minor C(sp2)–H activated product [PhCH2N2(NMe)C2(C6H3CF3)]RuCl(p-cymene) (2a′). Similar ruthenium complexes 2b, 2c, 2d and 2e with general formula RuCl2(p-cymene)(NHC) (NHC = MesCH2N2(NMe)C2Ph 2b, PhCH2N2(NMe)C2Ph 2c, TripCH2N2(NMe)C2Ph 2d, IMes 2e) were also synthesized. Subsequent reaction of Me3SiOSO2CF3 with 2a and 2b resulted in cationic ruthenium species [(PhCH2N2(NMe)C2(C6H4CF3))RuCl(p-cymene)][OSO2CF3] (3a) and [(MesCH2N2(NMe)C2Ph)RuCl(p-cymene)][OSO2CF3] (3b), respectively. Complexes 3a and 3b dissolved in CD3CN to give [(PhCH2N2(NMe)C2(C6H4CF3))RuCl(CD3CN)(p-cymene)][OSO2CF3] (4a) and [(MesCH2N2(NMe)C2Ph)RuCl(CD3CN)(p-cymene)][OSO2CF3] (4b), respectively. Cationic ruthenium species 4a and 4b failed to show catalytic activity towards hydrogenation of olefins. Ruthenium(II) complexes 2b–e with the general formula RuCl2(p-cymene)(NHC) were reacted with Et3SiH to generate a series of ruthenium(II) hydrides 5b–e. These compounds 5b–e are effective catalysts for the hydrogenation of terminal, internal and cyclic and functionalized olefins.

 Please wait while we load your content...
Please wait while we load your content...