Reversible CO exchange at platinum(0). An example of similar complex properties produced by ligands with very different stereoelectronic characteristics†
Abstract
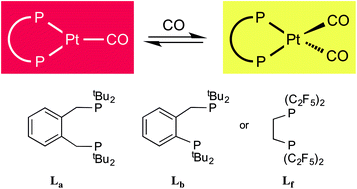
The ligands 1,2-C6H4(CH2PtBu2)2 (La) and 1,2-C6H4(PtBu2)(CH2PtBu2) (Lb) displace norbornene (nbe) from [Pt(η2-nbe)3] to give [PtL(η2-nbe)] where L = La (1a) or Lb (1b); 1a is fluxional on the NMR timescale. Reaction of 1a,b with CO gives the corresponding monocarbonyls [PtL(CO)] where L = La (2a) or Lb (2b) which then react further, and reversibly, to give the dicarbonyls [PtL(CO)2] where L = La (3a) or Lb (3b). The CO interchange between 2a,b and 3a,b is compared with the only other such system (2f and 3f), which are complexes of (C2F5)2PCH2CH2P(C2F5)2 (Lf). Ethene reacts smoothly with 2a to give (4a) and H2 with 2a generates some [PtH2(La)]. Protonation of 2a gives [Pt(La)(H)(CO)][B(C6F5)4] (5a) whose crystal structure has been determined. Similarly protonation of 2b gives [Pt(Lb)(H)(CO)][B(C6F5)4] as a mixture of geometric isomers 5b–6b.

 Please wait while we load your content...
Please wait while we load your content...