Syntheses, characterization, and structural studies of copper(i) complexes containing 1,1′-bis(di-tert-butylphosphino)ferrocene (dtbpf) and their application in palladium-catalyzed Sonogashira coupling of aryl halides†
Abstract
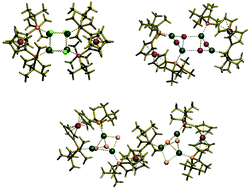
Four copper(I) complexes [Cu4(μ3-Cl)4(μ-dtbpf)2] (1), [Cu6(μ3-Br)4(μ2-Br)2(μ-dtbpf)3] (2), [Cu4(μ2-I)2(μ3-I)2(μ-dtbpf)2] (3) and [Cu2(μ1-CN)2(κ2-dtbpf)2] (4) were prepared using CuX (X = Cl, Br, I, CN) and 1,1′-bis(di-tert-butylphosphino)ferrocene (dtbpf) in 2 : 1 molar ratio in DCM–MeOH (50 : 50 V/V) at room temperature. These complexes were characterized by elemental analyses, IR, 1H and 31P NMR, ESI+-MS and electronic absorption spectroscopy. Molecular structures of the complexes 1, 2 and 3 were determined crystallographically. Complexes 1 and 3 are tetrameric Cu(I) pseudo-cubane like structures with a Cu4Cl4 core, and a stepped cubane-like structure with a Cu4I4 core, respectively, whereas complex 2 shows a trimeric copper(I) framework containing two [Cu3(μ3-Br)2(μ2-Br)] units that are bridged by three bidentate μ-dtbpf ligands. Each [Cu3(μ3-Br)2(μ2-Br)] unit forms a pyramid with one copper atom at the apex and one of the triangular faces capped by one bromine atom. All the complexes were found to be efficient catalysts for the Sonogashira reaction. The coupling products were obtained in moderate to good yields (58–99%) using Pd loadings of 0.2 mol% as well as complex loading of 0.1 mol%. The 31P NMR studies show that all the complexes are unstable during the course of tandem catalytic reaction and the dtbpf ligand migrates from copper(I) to palladium(II), which promotes palladium Sonogashira cross-coupling of activated and non-activated aryl halides.

 Please wait while we load your content...
Please wait while we load your content...