Lithium heterocuprates: the influence of the amido group on organoamidocuprate structures†
Abstract
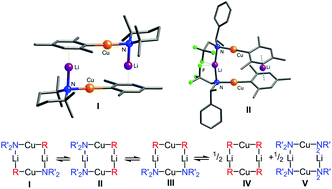
Lithium organoamidocuprates of the general stoichiometry LiCuR(NR′2) are an important class of organocopper reagents and have found widespread application in conjugate addition and other bond-forming reactions. The dependence of the structures and equilibrium of these species on the steric and electronic properties of the amido group is reported in both the solid state and in solution. Three different cuprate complexes have been crystallographically characterized: the organoamidocuprate [Cu2Li2Mes2TMP2] (2) (TMP = 2,2,6,6-tetramethylpiperidide) which is shown to adopt a head-to-tail conformation; [Cu2Li2(N(CH2Ph)CH2CH2NMe2)4] (3) which is a homoamidocuprate and contains additional coordination of the lithium centres from intra-molecular tertiary amine groups; and the diastereomeric organoamidocuprate [Cu2Li2Mes2(N(R-CH(Ph)Me)(CH2CF3))2] (4) which adopts a head-to-head conformation. Complex 4 is unique in being the first crystallographically characterised example of a head-to-head isomer of a heterocuprate, and its structure also has implications for the use of scalemic amidocuprates in asymmetrically induced conjugate addition. The solution equilibria of all new complexes have also been studied using 7Li NMR spectroscopy, and in each case the species observed in the crystal structure was shown to also be the predominant isomer in solution.
- This article is part of the themed collection: New Expeditions in Polar Organometallic Chemistry

 Please wait while we load your content...
Please wait while we load your content...