Homo- and heteroleptic alkoxycarbene f-element complexes and their reactivity towards acidic N–H and C–H bonds†
Abstract
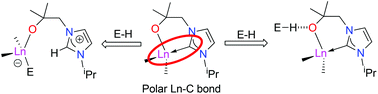
The reactivity of a series of organometallic rare earth and actinide complexes with hemilabile NHC-ligands towards substrates with acidic C–H and N–H bonds is described. The synthesis, characterisation and X-ray structures of the new heteroleptic mono- and bis(NHC) cyclopentadienyl complexes LnCp2(L) 1 (Ln = Sc, Y, Ce; L = alkoxy-tethered carbene [OCMe2CH2(1-C{NCHCHNiPr})]), LnCp(L)2 (Ln = Y) 2, and the homoleptic tetrakis(NHC) complex Th(L)44 are described. The reactivity of these complexes, and of the homoleptic complexes Ln(L)3 (Ln = Sc 3, Ce), with E–H substrates is described, where EH = pyrrole C4H4NH, indole C8H6NH, diphenylacetone Ph2CC(O)Me, terminal alkynes RC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH (R = Me3Si, Ph), and cyclopentadiene C5H6. Complex 1-Y heterolytically cleaves and adds pyrrole and indole N–H across the metal carbene bond, whereas 1-Ce does not, although 3 and 4 form H-bonded adducts. Complexes 1-Y and 1-Sc form adducts with CpH without cleaving the acidic C–H bond, 1-Ce cleaves the Cp–H bond, but 2 reacts to form the very rare H+–[C5H5]−–H+ motif. Complex 1-Ce cleaves alkyne C–H bonds but the products rearrange upon formation, while complex 1-Y cleaves the C–H bond in diphenylacetone forming a product which rearranges to the Y–O bonded enolate product.
CH (R = Me3Si, Ph), and cyclopentadiene C5H6. Complex 1-Y heterolytically cleaves and adds pyrrole and indole N–H across the metal carbene bond, whereas 1-Ce does not, although 3 and 4 form H-bonded adducts. Complexes 1-Y and 1-Sc form adducts with CpH without cleaving the acidic C–H bond, 1-Ce cleaves the Cp–H bond, but 2 reacts to form the very rare H+–[C5H5]−–H+ motif. Complex 1-Ce cleaves alkyne C–H bonds but the products rearrange upon formation, while complex 1-Y cleaves the C–H bond in diphenylacetone forming a product which rearranges to the Y–O bonded enolate product.
- This article is part of the themed collection: New Expeditions in Polar Organometallic Chemistry

 Please wait while we load your content...
Please wait while we load your content...