Synthesis of morphology controllable porous Co3O4 nanostructures with tunable textural properties and their catalytic application†
Abstract
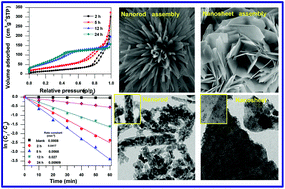
Porous cobalt oxide (Co3O4) nanorod (50–100 nm) and nanosheet-like (70–100 nm) particles were synthesized by a facile hydrothermal method at 150 °C for 2–5 h and 12–24 h, respectively, using aqueous-based precursors like cobalt nitrate, urea and water in the absence of any templating agents followed by their calcination at 300 °C. Morphology and textural properties were tuned by changing the synthesis time at 150 °C. A 3D architecture of Co3O4 was formed by the self-assembly of nanostructured (nanorod and nanosheet) particles. The BET surface area, pore volume and pore diameter of the sample prepared at 150 °C for 5 h were 112 m2 g−1, 0.5 cm3 g−1 and 7.4 nm, respectively, and it exhibited the highest catalytic performance with a rate constant of 56.8 × 10−3 min−1 for the degradation of Chicago Sky Blue 6B, a carcinogenic azo dye used in the textile, paper and food industries. Rod-like particles with a mesoporous structure rendered a better catalytic efficiency than sheet-like particles having both microporous and mesoporous structures. An interrelationship amongst the morphology, textural properties and the catalytic efficiency of Co3O4 was established.

 Please wait while we load your content...
Please wait while we load your content...