Cyclometalated Pd(ii) and Ir(iii) 2-(4-bromophenyl)pyridine complexes with N-heterocyclic carbenes (NHCs) and acetylacetonate (acac): synthesis, structures, luminescent properties and application in one-pot oxidation/Suzuki coupling of aryl chlorides containing hydroxymethyl†
Abstract
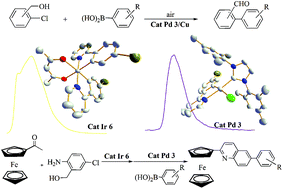
A series of cyclopalladated 2-(4-bromophenyl)pyridine (bpp) complexes [Pd(bpp)(NHC)Cl] 1–3, [Pd(bpp)(acac)] 4, cyclometalated iridium(III) complexes [Ir(bpp)2Cl]25 and [Ir(bpp)2(acac)] 6 have been synthesized and characterized. Their detailed structures have been determined by X-ray diffraction and many intermolecular C–H⋯X (Cl, Br, π) and π⋯π interactions were found in their crystals. Cyclometalated complexes 1–4 and 6 exhibit luminescence with emission peaks of 390–543 nm in dichloromethane solution under UV irradiation. Their application to coupling reactions of aryl chlorides containing hydroxymethyl was also investigated. An efficient 3/Cu cocatalyzed oxidation/Suzuki reaction for the synthesis of biarylaldehydes from chloro-phenylmethanol and arylboronic acids in air has been developed. In addition, a 6/3-cocatalyzed one-pot reaction of acetylferrocene, (2-amino-5-chlorophenyl)methanol, and arylboronic acids provided 6-aryl-2-ferrocenylquinolines in moderate to good yields.

 Please wait while we load your content...
Please wait while we load your content...