Three H2O2 molecules are involved in the “Fenton-like” reaction between Co(H2O)62+ and H2O2†
Abstract
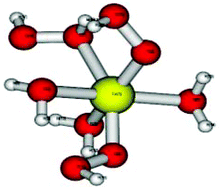
Co(II) complexes and Co(H2O)62+ are used as catalysts in advanced oxidation processes. Therefore it was decided to study the kinetics of reaction of Co(H2O)62+ with H2O2. Surprisingly, the kinetic results point out that the process involves three consecutive reactions, each of them requiring an H2O2 molecule, i.e. three H2O2 molecules ligate to the central cobalt cation prior to the formation of radicals. DFT analysis suggests that the transient (H2O)3CoII(OOH)2(H2O2) decomposes via: (H2O)3CoII(OOH)2(H2O2) → (H2O)3CoII(OOH)(˙OOH)(OH) + ˙OH ΔG0 = −5.975 kcal mol−1, with no evidence for the formation of a Co(III) transient. It is proposed that analogous mechanisms are involved whenever the redox potential of the central cation is too high to enable the reaction: M(H2O)6n+ + H2O2 → M(n+1)+aq + ˙OH + OH−.

 Please wait while we load your content...
Please wait while we load your content...