Aluminum complexes with bidentate amido ligands: synthesis, structure and performance on ligand-initiated ring-opening polymerization of rac-lactide†
Abstract
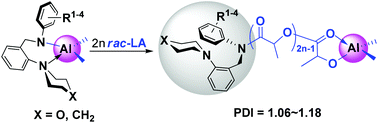
A series of mononuclear aluminum dimethyl complexes bearing bidentate N-[2-(1-piperidinyl)benzyl]anilino or N-(2-morpholinobenzyl)anilino ligands were synthesized via reactions of the corresponding proligands with trimethylaluminum upon heating, while at ambient temperature two trimethylaluminum adducts with neutral N-[2-(1-piperidinyl)benzyl]aniline proligands were obtained. These complexes were well characterized by NMR spectroscopy, elemental analysis and occasionally by EI-HRMS. The molecular structures of the typical trimethylaluminum adduct 2b and aluminum dimethyl complex 3b were further confirmed by X-ray diffraction studies. All the aluminum dimethyl complexes could effectively initiate the ring-opening polymerization (ROP) of rac-lactide in a well-controlled manner to afford PLAs with narrow molecular weight distributions (PDI = 1.06–1.18). The polymer samples obtained are systematically end-capped with the bidentate ancillary ligands as characterized by 1H NMR and ESI-TOF mass spectroscopy. Moreover, the introduction of substituent(s) at the ortho-position(s) of the anilino moiety in the ligand results in an obvious decrease in the catalytic activity of the corresponding aluminum complex, and complexes with meta-chloro substituted anilino units show higher activities likely due to the enhanced electrophilicity of the metal centers induced by the anilino groups.

 Please wait while we load your content...
Please wait while we load your content...