Remarkable anticancer activity of ferrocenyl-terpyridine platinum(ii) complexes in visible light with low dark toxicity†
Abstract
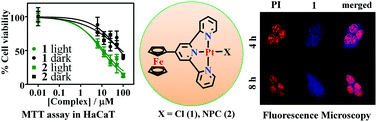
Ferrocenyl platinum(II) complexes (1–3), viz. [Pt(Fc-tpy)Cl]Cl (1), [Pt(Fc-tpy)(NPC)]Cl (2, HNPC = N-propargyl carbazole) and [Pt(Fc-bpa)Cl]Cl (3), were prepared, characterized and their anti-proliferative properties in visible light in human keratinocyte (HaCaT) cell lines have been studied. [Pt(Ph-tpy)Cl]Cl (4) was prepared and used as a control. Complexes 1 and 3, structurally characterized by X-ray crystallography, show distorted square-planar geometry for the platinum(II) centre. Complexes 1 and 2 having the Fc-tpy ligand showed an intense absorption band at ∼590 nm. The ferrocenyl complexes are redox active showing the Fc+–Fc couple near 0.6 V vs. SCE in DMF–0.1 M tetrabutylammonium perchlorate (TBAP). Complexes 1–3 showed external binding to calf thymus DNA. Both 1 and 2 showed remarkable photocytotoxicity in HaCaT cell lines giving respective IC50 values of 9.8 and 12.0 μM in visible light of 400–700 nm with low dark toxicity (IC50 >60 μM). Fluorescent imaging studies showed the spread of the complexes throughout the cell localising both in cytoplasm and the nucleus. The ferrocenyl complexes triggered apoptosis on light exposure as evidenced from the Annexin V-FITC/PI and DNA ladder formation assays. Spectral studies revealed the formation of ferrocenium ions upon photo-irradiation generating cytotoxic hydroxyl radicals via a Fenton type mechanism. The results are rationalized from a TDDFT study that shows involvement of ferrocene and the platinum coordinated terpyridine moiety as respective HOMO and LUMO.

 Please wait while we load your content...
Please wait while we load your content...