The dimeric nature of bonding in gallium: from small clusters to the α-gallium phase
Abstract
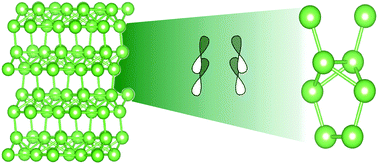
We consider the structural similarity of small gallium clusters to the bulk structure of α-gallium, which has been previously described as a molecular metal, via density functional theory-based computations. Previous calculations have shown that the tetramer, the hexamer, and the octamer of gallium are all structurally similar to the α-phase. We perform an analysis of the bonding in these clusters in terms of the molecular orbitals and atoms in molecules description in order to assess whether we can see similarities at these sizes to the bonding pattern, which is ascribed to the co-existence of covalent and metallic bonding in the bulk. The singlet Ga4 and Ga8 clusters can be constructed in a singlet ground state from the Ga-dimers in the first excited triplet state of the Ga2-molecule, the 3Σg− state. Molecular orbital (MO) analysis confirms that the dimer is an essential building block of these small clusters. Comparison of the AIM characteristics of the bonds within the clusters to the bonds in the bulk α-phase supports the identification of the covalent bond in the bulk as related to the 3Σg− state of the dimer.

 Please wait while we load your content...
Please wait while we load your content...