DFT computations support the σ-complex assisted metathesis (σ-CAM) mechanism for the 1,4-Rh shift of Cp*Rh(iii)–(η1-β-styryl) complexes†
Abstract
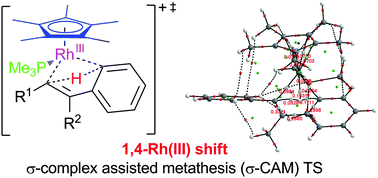
DFT calculations support the σ-complex assisted metathesis (σ-CAM) mechanism recently proposed for the first 1,4-Rh shift of a Rh(III) complex rather than the oxidative addition/reductive elimination pathway characteristic of Rh(I). A single, concerted TS (ΔG‡ = 27–34 kcal mol−1) was found and its electronic structure characterized by Bader's AIM analysis. The 4-centered TS is characterized by a enhanced charge separation (Rh and H atoms – positive, both C atoms – negative) relative to the σ-vinyl Rh starting material and the σ-aryl-Rh product. The AIM topological analysis of the electron density reveals a network of interactions: Rh with H as well as both Rh and H with both C(vinyl) and C(aryl) in the TS and confirms the C(vinyl)-Rh agnostic interaction observed experimentally in the σ-aryl-Rh product.

 Please wait while we load your content...
Please wait while we load your content...