Microscopic interactions of the imidazolium-based ionic liquid with molecular liquids depending on their electron-donicity
Abstract
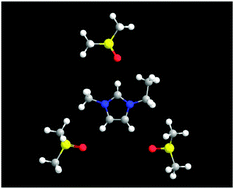
Microscopic interactions of an imidazolium-based ionic liquid, 1-ethyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide (C2mimTFSI), with dimethyl sulfoxide (DMSO), methanol (MeOH), and acetonitrile (AN) have been analyzed by means of Raman, attenuated total reflectance infrared (ATR-IR), 1H and 13C NMR spectroscopy techniques. The magnitude of the red-shift of the C2–H vibration mode of the imidazolium ring and the deshielding of the C2–H hydrogen and carbon atoms, compared with that of the other atoms of the ring or the anion, indicated a strong interaction between the C2–H hydrogen atom and the molecular liquids in the following order; DMSO ≫ MeOH > AN. This correlates with the order of the electron donicities of these molecular liquids which allows us to suggest a hydrogen bonding character of these interactions. The behavior of S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibration of DMSO as a function of the DMSO molar fraction xDMSO also suggested that DMSO molecules are stoichiometrically hydrogen-bonded with the three hydrogen atoms, C2,4,5–H, of the ring. In contrast, the hydrogen bonding between MeOH and the C4,5–H atoms is much weaker than that in DMSO. AN hardly forms hydrogen bonds with the C4,5–H atoms. Instead, AN molecules may interact with the imidazolium ring through the π–π interaction. The interactions between the imidazolium ring and the molecular liquids lead to the loosening of the TFSI anion from the cation; this correlates with both the blue-shift of the S
O vibration of DMSO as a function of the DMSO molar fraction xDMSO also suggested that DMSO molecules are stoichiometrically hydrogen-bonded with the three hydrogen atoms, C2,4,5–H, of the ring. In contrast, the hydrogen bonding between MeOH and the C4,5–H atoms is much weaker than that in DMSO. AN hardly forms hydrogen bonds with the C4,5–H atoms. Instead, AN molecules may interact with the imidazolium ring through the π–π interaction. The interactions between the imidazolium ring and the molecular liquids lead to the loosening of the TFSI anion from the cation; this correlates with both the blue-shift of the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of TFSI and the deshielding of the trifluoromethyl carbon atoms with an increase in the molar fraction of the molecular liquid xML. The latter is weak in the MeOH solutions, and may be explained by the possible hydrogen bonding of the MeOH hydroxyl group as an electron-acceptor with the TFSI anion. Furthermore, the organization of MeOH molecules around the ethyl and methyl groups of the cation is discussed in terms of the chemical shift of the hydrogen and carbon atoms in these groups as a function of xML.
O stretching vibration of TFSI and the deshielding of the trifluoromethyl carbon atoms with an increase in the molar fraction of the molecular liquid xML. The latter is weak in the MeOH solutions, and may be explained by the possible hydrogen bonding of the MeOH hydroxyl group as an electron-acceptor with the TFSI anion. Furthermore, the organization of MeOH molecules around the ethyl and methyl groups of the cation is discussed in terms of the chemical shift of the hydrogen and carbon atoms in these groups as a function of xML.

 Please wait while we load your content...
Please wait while we load your content...