Design of novel additives and nonaqueous solvents for lithium-ion batteries through screening of cyclic organic molecules: an ab initio study of redox potentials†
Abstract
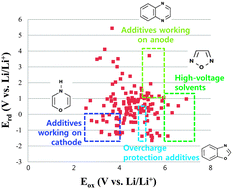
We have screened 142 cyclic organic compounds in search of novel functional additives and nonaqueous solvents for use in lithium-ion batteries through the use of ab initio calculations, and have determined redox potentials for all molecules. We have estimated the range of variation in oxidation potentials through heteroatom replacement and structure modification. By analyzing the oxidative properties of these compounds, we have shown that substituted heteroatoms and isomers in cyclic organic molecules can induce large variations of oxidation potentials more than 2.3 V. Calculations of reduction potentials show that the substituted heteroatoms, isomers, and the number of double bonds in cyclic organic molecules can change reduction potentials more than 1.7 V. Additionally, we have identified seventeen and twelve compounds that could serve as additives in eliciting film formation on cathodes and anodes, respectively. We have also identified five compounds that could function in the overcharge protection of over-lithiated layered oxide cathodes. Finally, we have identified five compounds that could serve as electrochemically stable solvents for high-voltage (>6 V vs. Li/Li+) applications. These inductive screenings and their analyses and findings extend our empirical knowledge into quantitative estimation in the design of materials.

 Please wait while we load your content...
Please wait while we load your content...