Why does bromine square palladium off? An ab initio study of brominated palladium and its nanomorphology†
Abstract
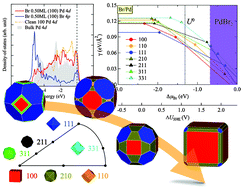
A first-principles description and prediction of brominated nanocrystals of Pd is presented. In particular, we conducted an extensive study of the adsorption behaviour of Br on various Pd surfaces (including both low and high Miller-index surfaces) as a function of its surface coverage. By coupling our calculated surface energies with ab initio (electrochemical) thermodynamics and the Gibbs–Wulff shape model, we find that the relative stability of the Pd surfaces is strongly modified by Br, allowing high Miller-index surfaces of Pd (namely the (210) surface) to become competitively favourable at moderate concentrations of Br. We also show that Pd nanoparticles assume a cube-like crystal shape at high concentrations of Br, exposing mainly the (100) facets with a Br surface coverage of 0.5 ML. This not only confirms and explains recent solution synthesis results, but also provides a quantitative atomic picture of the exposed surface facets, which is crucial in understanding the local surface chemistry of shape-controlled nanoparticles for better nanocatalyst design.

 Please wait while we load your content...
Please wait while we load your content...