Theoretical study on the initial stage of a magnesium battery based on a V2O5 cathode
Abstract
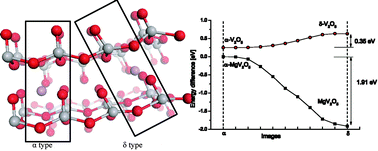
Several first-principles calculations based on density functional theory have been carried out looking at the key issues of a magnesium battery with a V2O5 cathode. This kind of magnesium battery was reported by D. Aurbach's group in 2013. Our theoretical studies provide explanations for the experimental findings such as higher voltage, slow ion diffusivity and the decrease of the crystallinity. The calculated open circuit voltage of a magnesium battery with a V2O5 cathode is 3.06 V, which is 0.22 V higher than a lithium battery with the same cathode. Electronic band structure calculations suggest that higher electronic conductivity must be expected in a magnesium battery. Elastic constants are obtained, which give information on the stability of the magnesiated cathode. Furthermore, we have also calculated the diffusion barriers of Li and Mg ions in the cathode using the nudged elastic band method. The hopping barrier of Mg ions is 1.26 eV, which is much higher than that of Li ions (0.35 eV). The obtained minimum energy paths show the different hopping processes in the lithium and magnesium batteries, which can explain the phenomenon of slow diffusion in experiments. The possible transition pathway between the α and δ phases is analyzed for the first time, which gives an explanation for the reversibility of Mg ions in the V2O5 cathode.

 Please wait while we load your content...
Please wait while we load your content...