Revisiting the role of exact exchange in DFT spin-state energetics of transition metal complexes
Abstract
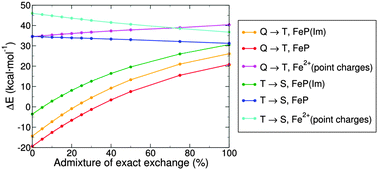
The effect of the exact exchange on the spin-state energetics of transition metal complexes is revisited with an attempt to clarify its origin and with regard to performance of DFT methods. Typically, by increasing an amount of the exact exchange in an exchange–correlation functional, higher spin states are strongly stabilized with respect to lower spin states. But this is not always the case, as revealed from the presented studies of heme and non-heme complexes, and of metal cations surrounded by point charges. It is argued that the sensitivity of the DFT spin-state energetics to the exact exchange admixture is rooted in the DFT description of the metal–ligand bonding rather than of the metal-centered exchange interactions. In the typical case, where transition from a lower spin state to a higher spin state involves an electron promotion from a nonbonding to an antibonding orbital, the lower spin state has a more delocalized charge distribution and contains a larger amount of nondynamical correlation energy than the higher spin state. However, DFT methods have problems with describing these two effects accurately. This interpretation allows us to explain why the exact exchange admixture has a much smaller effect on the energetics of spin transitions that involve only nonbonding d orbitals.
- This article is part of the themed collection: Density functional theory and its applications

 Please wait while we load your content...
Please wait while we load your content...