A mutarotation mechanism based on dual proton exchange in the amorphous d-glucose
Abstract
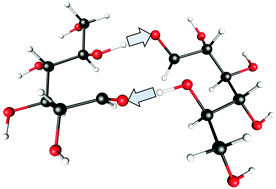
It is a well known fact that carbohydrates have unusual chemical and physical properties when they approach the glassy state during the cooling process. Differences between sugar aqueous solutions and their pure anhydrous states are caused mainly by the different intermolecular interactions related to the different hydrogen bond patterns. The mutarotation, a specific reaction in the saccharides, was recently investigated in the supercooled liquid and the glassy state of D-glucose. It was shown that the activation energy of this process in the supercooled liquid state is twice as low as for the same process in aqueous solution. In contrast, the activation energy in the glassy state is twice as high as in the aqueous solution. Herein, we present possible explanations for this phenomenon and propose a universal mechanism for the mutarotation process in the amorphous state of matter. In this work, for the first time, a double proton exchange mechanism in carbohydrates is proposed.

 Please wait while we load your content...
Please wait while we load your content...