Chemical and morphological changes during olivine carbonation for CO2 storage in the presence of NaCl and NaHCO3
Abstract
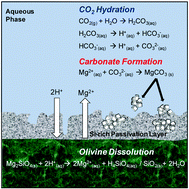
The increasing concentrations of CO2 in the atmosphere are attributed to the rising consumption of fossil fuels for energy generation around the world. One of the most stable and environmentally benign methods of reducing atmospheric CO2 is by storing it as thermodynamically stable carbonate minerals. Olivine ((Mg,Fe)2SiO4) is an abundant mineral that reacts with CO2 to form Mg-carbonate. The carbonation of olivine can be enhanced by injecting solutions containing CO2 at high partial pressure into olivine-rich formations at high temperatures, or by performing ex situ mineral carbonation in a reactor system with temperature and pressure control. In this study, the effects of NaHCO3 and NaCl, whose roles in enhanced mineral carbonation have been debated, were investigated in detail along with the effects of temperature, CO2 partial pressure and reaction time for determining the extent of olivine carbonation and its associated chemical and morphological changes. At high temperature and high CO2 pressure conditions, more than 70% olivine carbonation was achieved in 3 hours in the presence of 0.64 M NaHCO3. In contrast, NaCl did not significantly affect olivine carbonation. As olivine was dissolved and carbonated, its pore volume, surface area and particle size were significantly changed and these changes influenced subsequent reactivity of olivine. Thus, for both long-term simulation of olivine carbonation in geologic formations and the ex situ reactor design, the morphological changes of olivine during its reaction with CO2 should be carefully considered in order to accurately estimate the CO2 storage capacity and understand the mechanisms for CO2 trapping by olivine.

 Please wait while we load your content...
Please wait while we load your content...