Low-temperature conversion of titanate nanotubes into nitrogen-doped TiO2 nanoparticles
Abstract
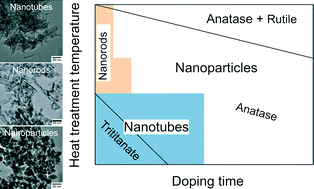
Hydrothermally synthesized protonated titanate nanotubes were doped with nitrogen using ammonia gas as the dopant. Thermal decomposition of urea, which served as the ammonia source, offered a low-temperature synthesis route for obtaining a potential visible-light photocatalyst. Nitrogen doping could be achieved at as low as 200 °C. The doped samples were calcined at different temperatures and changes in the morphology and crystalline phase were studied by transmission and scanning electron microscopy, selected area electron diffraction, energy-dispersive X-ray spectroscopy and X-ray diffraction. The nitrogen content and calcination temperature were found to affect the size and shape of the particles as well as their crystalline phase to a great extent. H-form trititanate was shown to transform into rutile TiO2 through the anatase phase in parallel with the collapse of the nanotube morphology and the production of rod-like nanoparticles first and then finally round nitrogen-doped nanoparticles. A phase map was constructed from the data to facilitate the rational design of N-doped trititanate nanotube-based nanostructures.

 Please wait while we load your content...
Please wait while we load your content...