Template-free solvothermal synthesis of WO3/WO3·H2O hollow spheres and their enhanced photocatalytic activity from the mixture phase effect†
Abstract
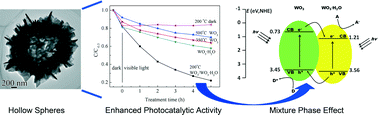
Well-defined WO3·H2O hollow spheres composed of nanoflakes were successfully synthesized by a template-free solvothermal process with i-PrOH–H2O mixture solvent. In this process, the tungsten precursor was firstly hydrolyzed to form solid spheres composed of both WO3·2H2O and WO3·H2O phases. Then, these solid spheres underwent an Ostwald ripening process and the WO3·2H2O phase was dehydrated to WO3·H2O at the same time to form hollow spheres with a pure WO3·H2O phase. With appropriate calcination temperature, hollow spheres with WO3 (major) and WO3·H2O (minor) mixture phases were created. These hollow spheres with WO3 and WO3·H2O mixture phases demonstrated a largely enhanced photocatalytic activity for RhB degradation under visible light irradiation compared with either pure WO3·H2O hollow spheres or pure WO3 hollow spheres which could be attributed to the matched band structure between the WO3 and WO3·H2O phases. Thus, an effective charge carrier separation can occur between the WO3 and WO3·H2O phases of these hollow spheres under visible light irradiation, contributing to the observed largely enhanced photocatalytic performance.

 Please wait while we load your content...
Please wait while we load your content...