Synthon polymorphs of 1 : 1 co-crystal of 5-fluorouracil and 4-hydroxybenzoic acid: their relative stability and solvent polarity dependence of grinding outcomes†
Abstract
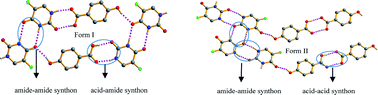
Although polymorphism is a common phenomenon, the polymorphism of co-crystals is not studied extensively as compared to single-component molecules. Herein we report polymorphism in a co-crystal system comprising 5-fluorouracil, and 4-hydroxybenzoic acid with a 1 : 1 stoichiometry. The polymorphs were characterized by single-crystal and powder X-ray diffraction, differential scanning calorimetry and thermogravimetric analysis. Crystal structure analysis revealed different synthons of 5-fluorouracil and 4-hydroxybenzoic acid in two forms. The solvent-drop grinding experiments show a high degree of solvent polarity specificity. The theoretical and experimental methods suggest an enantiotropic relationship between the polymorphs.
- This article is part of the themed collection: International Year of Crystallography Celebration: Asia-Pacific

 Please wait while we load your content...
Please wait while we load your content...