Strengthening N⋯X halogen bonding via nitrogen substitution in the aromatic framework of halogen-substituted arylpyrazinamides†
Abstract
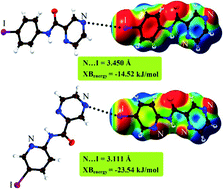
The importance of N⋯X halogen bonding in a series of N-(5-halo-2-pyridinyl)pyrazine-2-carboxamides has been investigated by different methods. The results show that when nitrogen is substituted for carbon in the aryl backbone of the parent compound, it can affect the electron accepting ability of bromine and iodine substituents. Thus, a stronger halogen bond can be formed.

 Please wait while we load your content...
Please wait while we load your content...