Highly selective directed arylation reactions via back-to-back dehydrogenative C–H borylation/arylation reactions†
Abstract
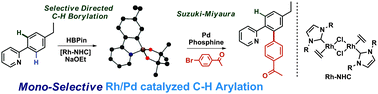
Dimeric rhodium N-heterocyclic carbene complexes are demonstrated to be effective catalyst precursors for directed C–H borylation reactions at room temperature. The reactions are highly selective for mono-borylation and can be combined with a one-pot Suzuki–Miyaura coupling to give C–H arylation products with exclusive selectivity for mono-arylation without the requirement for steric blocking groups.

 Please wait while we load your content...
Please wait while we load your content...