B(C6F5)3 promoted cyclisation of internal propargyl esters: structural characterisation of 1,3-dioxolium compounds†
Abstract
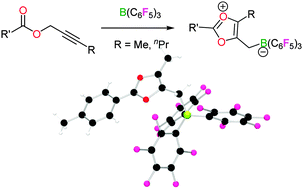
The reactions of internal propargyl esters with B(C6F5)3 provide access to stable zwitterionic 1,3-dioxolium compounds which are characterised by X-ray diffraction. These 6π-electron systems show no significant aromatic stabilisation.

 Please wait while we load your content...
Please wait while we load your content...