The versatility of furfuryl alcohols and furanoxonium ions in synthesis†
Abstract
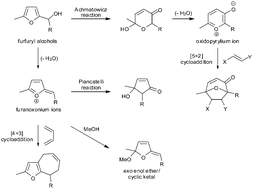
Substituted furfuryl alcohols are extraordinarily versatile starting materials in synthesis. They are precursors to furanoxonium ion intermediates which are implicated in the Piancatelli reaction (leading to 2-cyclopentenones) and in the synthesis of novel dihydrofuran-based exo enol ether/cyclic ketal natural products. They are also intermediates in a recently discovered (4+3) cycloaddition reaction with 1,3-dienes leading to furan ring-fused cycloheptenes. Here we provide a perspective on recent developments in these areas of synthesis, alongside recent applications of the Achmatowicz reaction and [5+2] cycloaddition reactions of the resulting oxidopyrylium ions.
- This article is part of the themed collection: In Celebration of Richard Taylor’s 65th Birthday

 Please wait while we load your content...
Please wait while we load your content...