Counterion effects in the catalytic stereoselective synthesis of 2,3′-pyrrolidinyl spirooxindoles†
Abstract
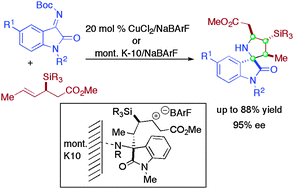
A Lewis acid-catalyzed stereoselective [3+2] annulation of crotylsilanes with iminooxindoles is reported to access 2,3′-pyrrolidinyl spirooxindoles with four stereocenters. The addition of NaBArF significantly enhances reactivity, allowing either metal salts or acidic clay to be effective catalysts for the stereoselective reaction.
- This article is part of the themed collection: 2014 Emerging Investigators

 Please wait while we load your content...
Please wait while we load your content...