The STIL protein contains intrinsically disordered regions that mediate its protein–protein interactions†
Abstract
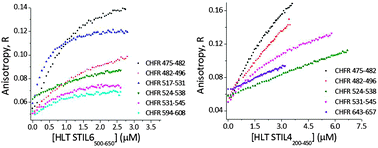
The STIL protein participates in mitosis and malignant transformation by regulating centrosomal duplication. Using biophysical methods we studied the structure and interactions of STIL. We revealed that its central domain is intrinsically disordered and mediates protein–protein interactions of STIL. The intrinsic disorder may provide STIL with the conformational flexibility required for its multitude binding.
- This article is part of the themed collection: 2014 Emerging Investigators

 Please wait while we load your content...
Please wait while we load your content...