A validated liquid chromatographic method for the determination of fluphenazine hydrochloride in the presence of its degradation products: application to degradation kinetics
Abstract
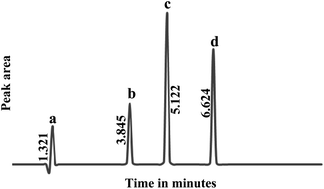
A simple, stability-indicating, reversed phase liquid chromatographic method has been developed for the determination of fluphenazine hydrochloride in the presence of its oxidative and ultraviolet degradation products. Reversed phase chromatography was conducted using an ODS C18 (150 × 4.6 mm id) column at ambient temperature with UV-detection at 250 nm. A mobile phase consisting of 0.03 M potassium dihydrogen phosphate buffer–acetonitrile (40 : 60, v/v) adjusted to pH 4 was used for the separation of the studied drug and its degradation products at a flow rate of 1 mL min−1. The method showed a good linearity over the concentration range of 2.0–20.0 μg mL−1 with a detection limit (LOD) of 0.8 μg mL−1 and a quantification limit (LOQ) of 1.5 μg mL−1. The proposed method was successfully validated and applied for the analysis of the drug in its commercial dosage forms; the obtained results were favorably compared with those obtained by the official and comparison methods. Moreover, the method was utilized to investigate the kinetics of the oxidative degradation of the drug. The first-order rate constant, half-life time, and activation energy of the degradation were calculated.

 Please wait while we load your content...
Please wait while we load your content...