1,3-γ-Silyl-elimination in electron-deficient cationic systems†
Abstract
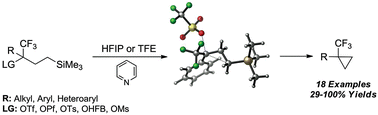
Placement of an electron-withdrawing trifluoromethyl group (–CF3) at a putative cationic centre enhances γ-silyl neighbouring-group participation (NGP). In stark contrast to previously studied γ-silyl-substituted systems, the preferred reaction pathway is 1,3-γ-silyl elimination, giving ring closure over solvent substitution or alkene formation. The scope of this cyclopropanation reaction is explored for numerous cyclic and acyclic examples, proving this method to be a viable approach to preparing CF3-substituted cyclopropanes and bicyclic systems, both containing quaternary centres. Rate-constants, kinetic isotope effects, and quantum mechanical calculations provided evidence for this enhancement and further elaborated the disparity in the reaction outcome between these systems and previously studied γ-silyl systems.

 Please wait while we load your content...
Please wait while we load your content...