Controlling selectivity in the reductive activation of CO2 by mixed sandwich uranium(iii) complexes†
Abstract
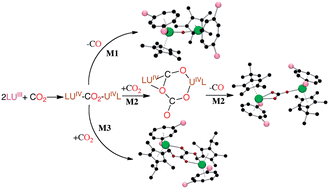
The synthesis and molecular structures of a range of uranium(III) mixed sandwich complexes of the type [U(η8-C8H6(1,4-SiMe3)2)(η5-CpMe4R)] (R = Me, Et, iPr, tBu) and their reactivity towards CO2 are reported. The nature of the R group on the cyclopentadienyl ring in the former has a significant effect on the outcome of CO2 activation: when R = Me, the products are the bridging oxo complex {U[η8-C8H6(1,4-SiMe3)2](η5-CpMe5)}2(μ-O) and the bridging oxalate complex {U[η8-C8H6(1,4-SiMe3)2](η5-CpMe5)}2(μ-η2:η2-C2O4); for R = Et or iPr, bridging carbonate {U[η8-C8H6(1,4-SiMe3)2](η5-CpMe4R)}2(μ-η1:η2-CO3) and bridging oxalate complexes {U[η8-C8H6(1,4-SiMe3)2](η5-CpMe4R)}2(μ-η2:η2-C2O4) are formed in both cases; and when R = tBu the sole product is the bridging carbonate complex {U[η8-C8H6(1,4-SiMe3)2](η5-CpMe4tBu)}2(μ-η1:η2-CO3). Electrochemical studies on both the uranium(III) complexes and the dimeric uranium(IV) CO2 reduction products have been carried out and all exhibit quasi reversible redox processes; in particular, the similarities in the U(III)/U(IV) redox couples suggest that the selectivity in the outcome of CO2 reductive activation by these complexes is steric in origin rather than electronic. The latter conclusion is supported by a detailed computational DFT study on the potential mechanistic pathways for reduction of CO2 by this system.

 Please wait while we load your content...
Please wait while we load your content...