Conformationally strained trans-cyclooctene with improved stability and excellent reactivity in tetrazine ligation†
Abstract
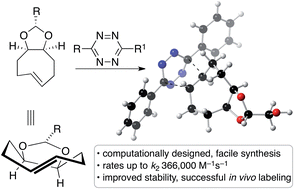
Computation has guided the design of conformationally-strained dioxolane-fused trans-cyclooctene (d-TCO) derivatives that display excellent reactivity in the tetrazine ligation. A water soluble derivative of 3,6-dipyridyl-s-tetrazine reacts with d-TCO with a second order rate k2 366 000 (±15 000) M−1 s−1 at 25 °C in pure water. Furthermore, d-TCO derivatives can be prepared easily, are accessed through diastereoselective synthesis, and are typically crystalline bench-stable solids that are stable in aqueous solution, blood serum, or in the presence of thiols in buffered solution. GFP with a genetically encoded tetrazine-containing amino acid was site-specifically labelled in vivo by a d-TCO derivative. The fastest bioorthogonal reaction reported to date [k2 3 300 000 (±40 000) M−1 s−1 in H2O at 25 °C] is described herein with a cyclopropane-fused trans-cyclooctene. d-TCO derivatives display rates within an order of magnitude of these fastest trans-cyclooctene reagents, and also display enhanced stability and aqueous solubility.

 Please wait while we load your content...
Please wait while we load your content...