Oximes as reversible links in polymer chemistry: dynamic macromolecular stars†
Abstract
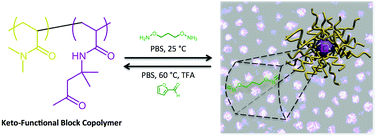
We demonstrate the formation of oxime-functional macromolecular stars that are able to dissociate and reconstruct themselves upon application of a stimulus. The reversible nature of the oxime bond in the presence of externally added alkoxyamines or carbonyl compounds enables reconfiguration via competitive exchange. Reversible addition–fragmentation chain transfer (RAFT) polymerization was utilized to prepare well-defined amphiphilic block copolymers in which a hydrophobic keto-functional block allowed self-assembly into micelles in water. Adding a difunctional alkoxyamine small molecule to these solutions resulted in crosslinking of the micelles to yield macromolecular stars. The reversible nature of the O-alkyl oxime linkages was demonstrated via competitive exchange with excess of carbonyl compounds or monofunctional alkoxyamine under acidic conditions and at elevated temperatures to result in dissociation of the stars to unimolecular oxime-functional polymer chains.

 Please wait while we load your content...
Please wait while we load your content...