Development of a polymer theranostic for prostate cancer†
Abstract
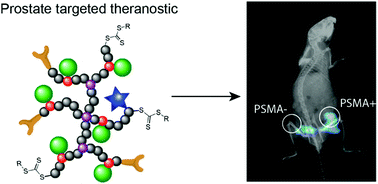
Theranostics offers an improved treatment strategy for prostate cancer by facilitating simultaneous targeting of tumour cells with subsequent drug delivery and imaging. In this report we describe the synthesis of hyperbranched polymers that are biocompatible, can specifically target and be internalised by prostate cancer cells (through targeting of prostate-specific membrane antigen – PSMA) and ultimately facilitate controlled delivery of a model drug. The theranostic also incorporates a far-red fluorescent dye that allows tracking of the polymer via optical imaging. Controlled synthesis of the polymer is achieved via reversible addition fragmentation chain transfer polymerisation of polyethylene glycol monomethyl methacrylate, with ethylene glycol dimethacrylate as the branching agent. Incorporation of 20 mol% of an hydrazide-methacrylate monomer allows post-ligation of a model drug, fluorene-2-carboxaldehyde, through a hydrolytically-degradable hydrazone linkage. The rate of degradation of this particular linker was enhanced at endosomal pH (pH = 5.5) where ∼95% of the model drug was released in 4 hours compared to less than 5% released over the same period at physiological pH. The theranostic showed high uptake into prostate cancer cells expressing prostate-specific membrane antigen, while minimal uptake was observed in PC3 cells negative for PSMA, highlighting the enhanced efficacy of the targeting ligand.

 Please wait while we load your content...
Please wait while we load your content...