Synthesis and immunological effects of heroin vaccines†
Abstract
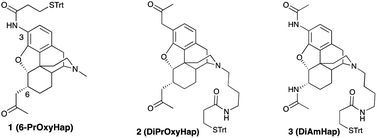
Three haptens have been synthesized with linkers for attachment to carrier macromolecules at either the piperidino-nitrogen or via an introduced 3-amino group. Two of the haptens, with a 2-oxopropyl functionality at either C6, or at both the C3 and C6 positions on the 4,5-epoxymorphinan framework, as well as the third hapten (DiAmHap) with diamido moieties at both the C3 and C6 positions, should be much more stable in solution, or in vivo in a vaccine, than a hapten with an ester in one of those positions, as found in many heroin-based haptens. A “classical” opioid synthetic scheme enabled the formation of a 3-amino-4,5-epoxymorphinan which could not be obtained using palladium chemistry. Our vaccines are aimed at the reduction of the abuse of heroin and, as well, at the reduction of the effects of its predominant metabolites, 6-acetylmorphine and morphine. One of the haptens, DiAmHap, has given interesting results in a heroin vaccine and is clearly more suited for the purpose than the other two haptens.

 Please wait while we load your content...
Please wait while we load your content...