Palladium-catalyzed carbonylative addition of aryl bromides to arylalkynes: a simple and efficient method for chalcone synthesis†
Abstract
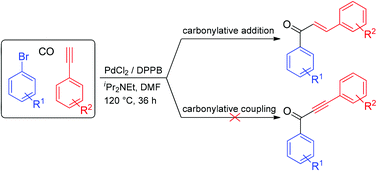
Palladium-catalyzed carbonylative addition of aryl bromides to terminal arylalkynes was carried out to produce chalcones in satisfactory to excellent yields. The unprecedented carbonylation reaction proceeded smoothly under mild conditions in the presence of a simple palladium catalyst system (PdCl2/DPPB/iPr2NEt) in N,N-dimethyl formamide.

 Please wait while we load your content...
Please wait while we load your content...