Pd-catalyzed carbonylation for the construction of tertiary and quaternary carbon centers with sp3 carbon partners†
Abstract
The first examples of a Pd-catalyzed carbonylation of aryl boronic acids with sp3 carbon partners are presented. Various boronic acids were shown to react with 1,3-diesters and 1,3-diketones to afford structurally unique carbonyl compounds. By employing 2-substituted 1,3-diesters, synthetically-challenging quaternary carbon centres were accessed. In total, 42 examples of aryl carbonyl compounds were prepared in moderate to good yields. The catalytic system features the use of a bidentated phosphine ligand and a relatively low CO pressure (5 atm), providing an easy, alternative method for the preparation of triketones.
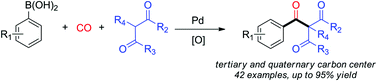

 Please wait while we load your content...
Please wait while we load your content...