CuI-catalyzed cycloisomerization of propargyl amides†
Abstract
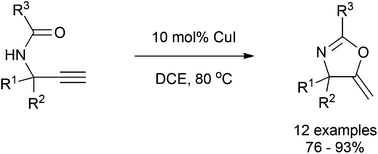
The synthesis of substituted dihydrooxazoles by the CuI-catalyzed cycloisomerization of terminal propargyl amides is reported. The reaction has been shown to have good substrate scope and experiments to delineate the mechanism have been performed. Substrates containing a benzylic methylene were oxidized to the ketone under the reaction conditions.

 Please wait while we load your content...
Please wait while we load your content...