Design, synthesis and evaluation of novel tacrine–rhein hybrids as multifunctional agents for the treatment of Alzheimer's disease†
Abstract
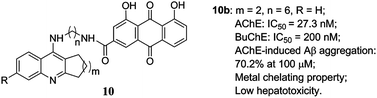
A series of tacrine–rhein hybrid compounds have been designed and synthesized as novel multifunctional potent ChE inhibitors. Most of the compounds inhibited ChEs in the nanomolar range in vitro effectively. Compound 10b was one of the most potent inhibitors and was 5-fold more active than tacrine toward AChE, and it also showed a moderate BuChE inhibition with an IC50 value of 200 nM. Kinetic and molecular modeling studies of 10b also indicated that it was a mixed-type inhibitor binding simultaneously to the active and peripheral sites of AChE. In inhibition of the AChE-induced Aβ aggregation assay, compound 10b (70.2% at 100 μM) showed the greatest inhibitory activity. In addition, 10b showed metal-chelating property and low hepatotoxicity. These results suggested that 10b might be an excellent multifunctional agent for AD treatment.

 Please wait while we load your content...
Please wait while we load your content...