Dynamics of Gal80p in the Gal80p–Gal3p complex differ significantly from the dynamics in the Gal80p–Gal1p complex: implications for the higher specificity of Gal3p†
Abstract
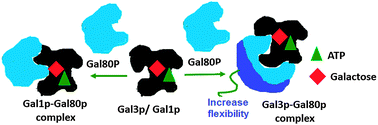
The expression of the GAL gene in Sacharomyces cerevisiae is regulated by three proteins; Gal3p/Gal1p, Gal80p and Gal4p. Both Gal3p and Gal1p act as transcriptional inducers, though Gal3p has a higher activity than Gal1p. The difference in activity may depend on the strength of the interaction and dynamical behavior of these proteins during complex formation with the repressor protein Gal80p. To address these queries we have modeled the binding interface of the Gal1p–Gal80p and Gal3p–Gal80p complexes. The comparison of the dynamics of these proteins in the complex and in the Apo protein was carried out. It was observed that the binding of Gal3p with Gal80p induces significant flexibility in Gal80p on a surface different from the one involved in binding with Gal3p. Several other differences at the interface between the Gal3p–Gal80p and the Gal1p–Gal80p complex were observed, which might permit Gal3p to act as a transcriptional inducer with higher activity. Further, we have discussed the dynamical event and plausible mechanism of complex formation of Gal3p and Gal1p with Gal80p at the molecular level.

 Please wait while we load your content...
Please wait while we load your content...