Self-oxidation of cytochrome c at methionine80 with molecular oxygen induced by cleavage of the Met–heme iron bond†
Abstract
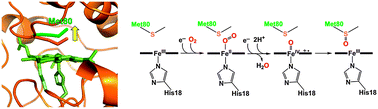
Met80 of cytochrome c (cyt c) has been shown to dissociate from its heme iron when cyt c interacts with cardiolipin (CL), which triggers the release of cyt c into the cytosol initiating apoptosis. We found that the mass of human cyt c increases by 16 Da in the Met80–Lys86 region by reaction with molecular oxygen in the presence of CL-containing liposomes and dithiothreitol (DTT). To investigate the effect of Met80 dissociation on the reaction of cyt c with molecular oxygen without affecting its secondary structures, a human cyt c mutant (Δ8384 cyt c) was constructed by removing two amino acids (Val83 and Gly84) from the loop containing Met80. According to MALDI-TOF-MS and tandem mass measurements, Met80 of Δ8384 cyt c was modified site-specifically to methionine sulfoxide when purified in the presence of molecular oxygen, whereas Met80 was not modified in the absence of molecular oxygen. A red-shift of the Soret band from 406 to 412 nm and absorption increase at ∼536 and ∼568 nm were observed for Δ8384 cyt c when it reacted with DTT and molecular oxygen, followed by a further red-shift of the Soret band to 416 nm and absorption increase at ∼620 and ∼650 nm. These results indicate that Met80 of cyt c is oxidized site-specifically by formation of the oxy and subsequent compound I-like species when Met80 dissociates from the heme iron, where the Met80 modification may affect its peroxidase activity related to apoptosis.

 Please wait while we load your content...
Please wait while we load your content...