Effects of pseudophosphorylation mutants on the structural dynamics of smooth muscle myosin regulatory light chain†
Abstract
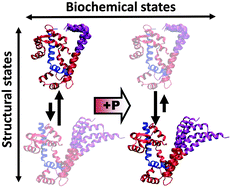
We have performed 50 independent molecular dynamics (MD) simulations to determine the effect of pseudophosphorylation mutants on the structural dynamics of smooth muscle myosin (SMM) regulatory light chain (RLC). We previously showed that the N-terminal phosphorylation domain of RLC simultaneously populates two structural states in equilibrium, closed and open, and that phosphorylation at S19 induces a modest shift toward the open state, which is sufficient to activate smooth muscle. However, it remains unknown why pseudophosphorylation mutants poorly mimic phosphorylation-induced activation of SMM. We performed MD simulations of unphosphorylated, phosphorylated, and three pseudophosphorylated RLC mutants: S19E, T18D/S19D and T18E/S19E. We found that the S19E mutation does not shift the equilibrium toward the open state, indicating that simple charge replacement at position S19 does not mimic the activating effect of phosphorylation, providing a structural explanation for previously published functional data. In contrast, mutants T18D/S19D and T18E/S19E shift the equilibrium toward the open structure and partially activate in vitro motility, further supporting the model that an increase in the mol fraction of the open state is coupled to SMM motility. Structural analyses of the doubly-charged pseudophosphorylation mutants suggest that alterations in an interdomain salt bridge between residues R4 and D100 results in impaired signal transmission from RLC to the catalytic domain of SMM, which explains the low ATPase activity of these mutants. Our results demonstrate that phosphorylation produces a unique structural balance in the RLC. These observations have important implications for our understanding of the structural aspects of activation and force potentiation in smooth and striated muscle.

 Please wait while we load your content...
Please wait while we load your content...