Porphyrin–cobaloxime complexes for hydrogen production, a photo- and electrochemical study, coupled with quantum chemical calculations†
Abstract
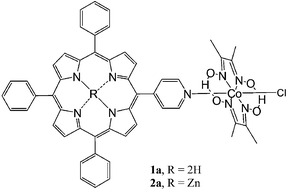
Two porphyrin–cobaloxime complexes; [{Co(dmgH)2Cl}{MPyTPP}] (1a) and [{Co(dmgH)2Cl}{ZnMPyTPP}] (2a) (dmgH = dimethylglyoxime, MPyTPP = 5-(4-pyridyl)-10,15,20-triphenylporphyrin) have been synthesised as model systems for the generation of hydrogen from water. Although initially envisaged as photocatalytic systems neither complex catalysed the reduction of water to hydrogen following irradiation. However, both complexes are molecular precursors for hydrogen evolution under electrochemical conditions. Turnover numbers for hydrogen production of 1.8 × 103 and 5.1 × 103 were obtained for 1a and 2a respectively following potentiostatic electrolysis at −1.2 V vs. Ag/AgCl while cobaloxime alone produced a turnover-number of 8.0 × 103. The photophysical properties of 1a and 2a were examined to provide an explanation for the lack of photochemical activity. These results, coupled with quantum chemical calculations, confirm that porphyrins fail to act as light-harvesting units for these systems and that the lowest energy excited states are in fact cobaloxime-based rather than porphyrin based.

 Please wait while we load your content...
Please wait while we load your content...