Palladium-catalyzed amination of meso-(bromophenyl)porphyrins with diamines and azamacrocycles†
Abstract
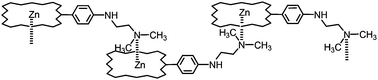
Novel diamino and azamacrocycle functionalized porphyrins were efficiently synthesized by palladium-catalyzed amination of mono- and bis(meso-(bromophenyl))porphyrins. The optimization of reaction conditions allowed us to achieve high yields of products with substrates of different types. Supramolecular utility of the thus obtained aminoporphyrins was shown by investigations of processes of coordination self-assembly in solution by NMR and UV-Vis spectroscopy. The crystalline 1D-coordination polymer formed via self-assembly of N,N-dimethylethylenediamine substituted zinc porphyrin was characterized by X-ray diffraction.

 Please wait while we load your content...
Please wait while we load your content...